|
|
 EasyMax GDH Test StripsFDA approves Oak Tree International Holdings, Inc. / EPS Bio Technology Corp’s GDH-FAD strips and meter for individual and multiple patients use. Oak Tree International Holdings, Inc. / EPS Bio Technology Corp. announced today that the US Food and Drug Administration (FDA) has approved our GDH-FAD blood glucose monitoring system for quantitative measurement of glucose in venous whole blood and fresh capillary whole blood. This technology will allow for more accurate readings (+15%) per FDA guidelines and also detects under-fill in the strips. Our GDH-FAD blood glucose monitoring system for multiple patients use is the only FDA approved currently in the market. Our GDH-FAD blood glucose monitoring system is intended for use at home (over the counter “OTC”) by a single patient with diabetes and should not be shared as an aid to monitor the effectiveness of diabetes control or for multiple patients use in professional healthcare settings as an aid to monitor the effectiveness of diabetes control. The system is not to be used on neonates nor for the diagnosis of or screening for diabetes mellitus. Alternate site testing can only be used during steady state blood glucose conditions. The system is only used with single use lancing devices. Oak Tree International Holdings, Inc. / EPS Bio Technology Corp is a world class manufacturer of diabetes care products. With customers all over Europe, Asia, Middle East and USA, our innovation, quality and service offers an unparalleled choice in diabetes care management. Oak Tree International Holdings, Inc. / EPS Bio Technology Corp is a global company with its factory in Hsinchu City, Taiwan and headquarter located in Las Vegas, NV. 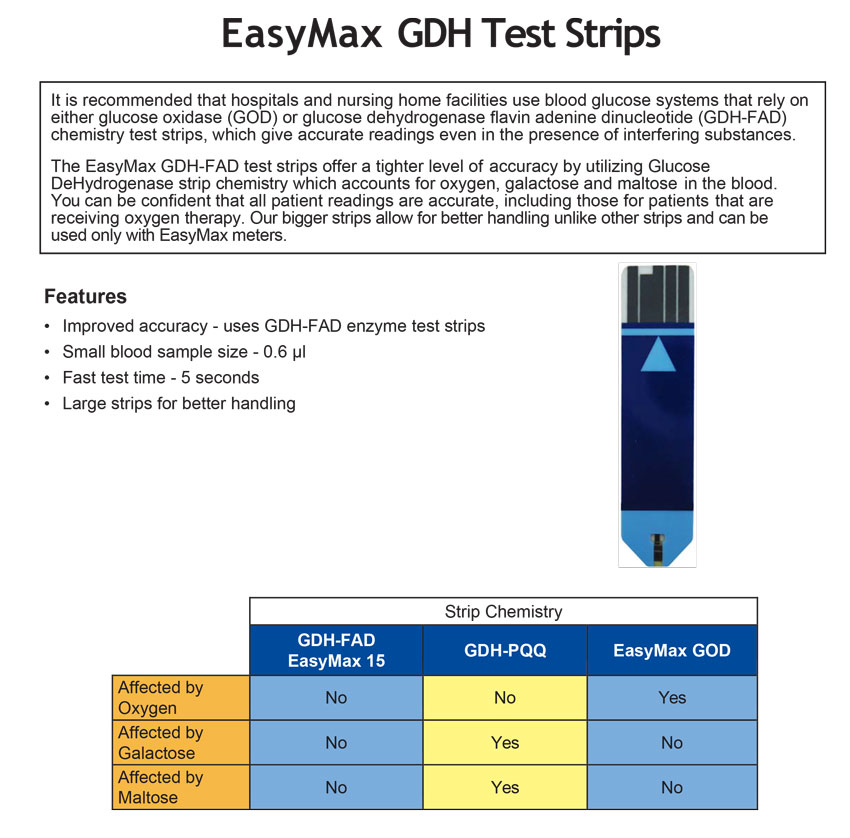
|








